Recognised International Standards
We have designed Conforma to meet international standards for regulatory systems.
For example, medicine applications can be submitted in line with the internationally agreed format (Common Technical Document format).
Below are some examples of where we have also incorporated standardised data tables for terms and definitions:
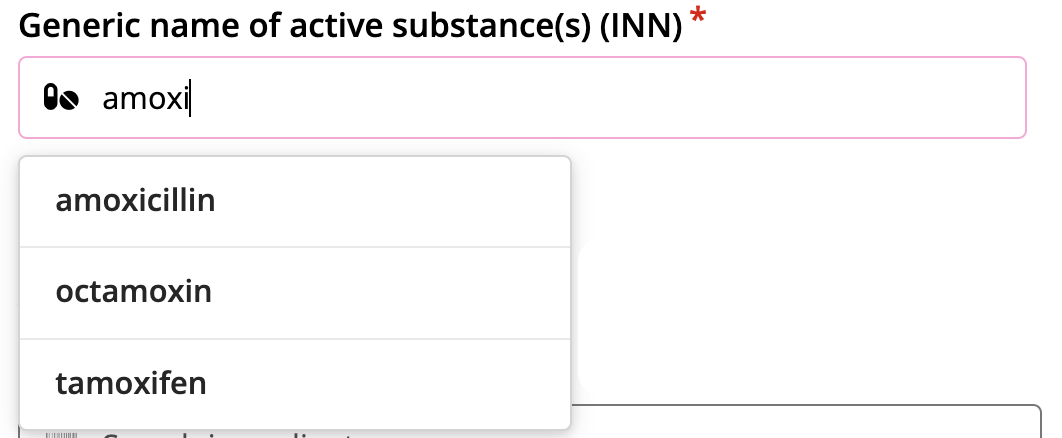
Ingredient names
Using the International Nonproprietary Names (INN) for the identification of pharmaceutical substances or active pharmaceutical ingredients.
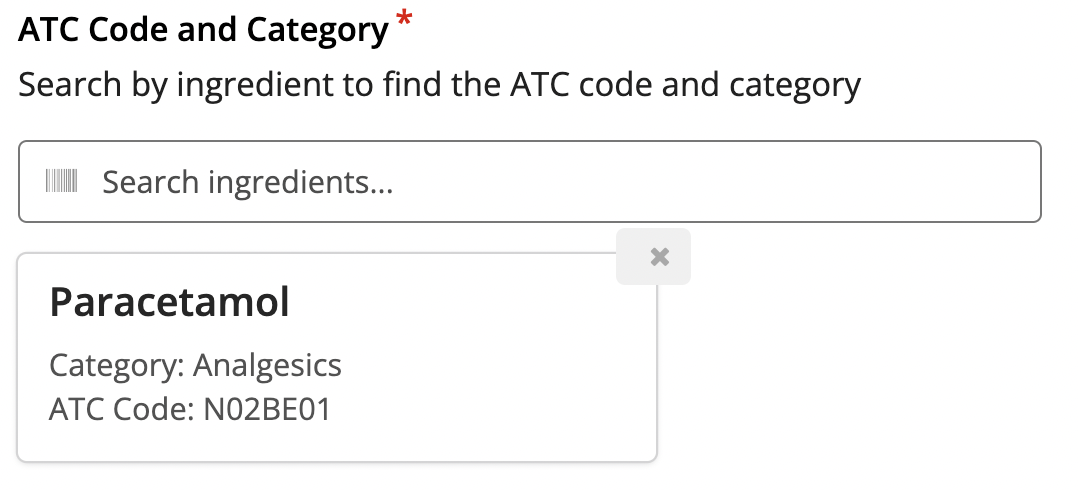
ATC Classification
Capturing the products using the Anatomical Therapeutic Chemical (ATC) classification system.
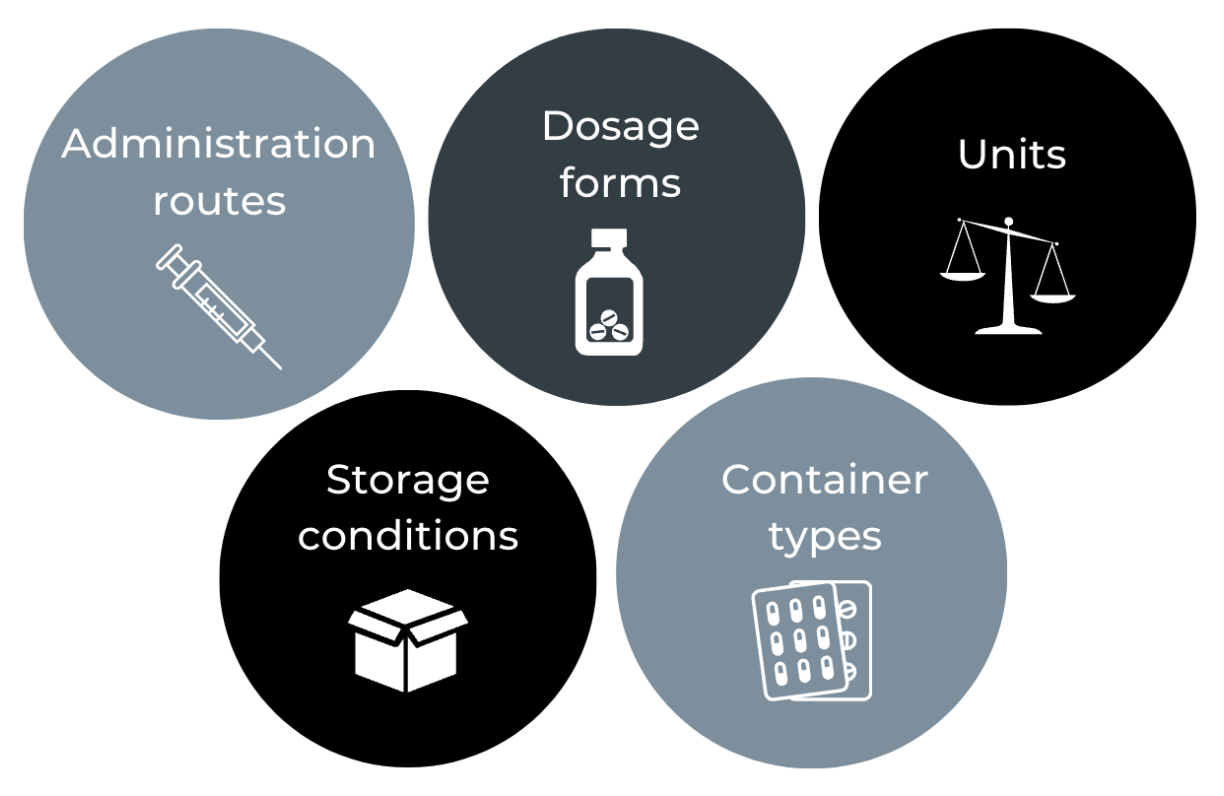
Access to standard datasets
For medicines registration processing, we have incorporated data tables for dose forms, routes and methods of administration, containers, closures, administration devices and units of presentation to help improve data quality and sharing.
You can also incorporate your own data and standards if needed.
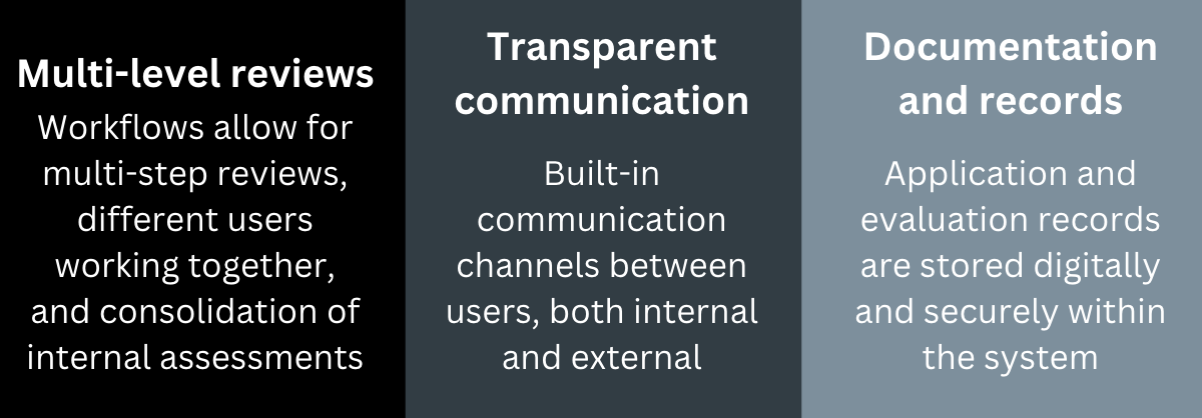
Good Review Practices
Features to encourage Good Review Practices are built into the system.