Reviewing Applications
This guide outlines how to review a medicine application in the Conforma Demo as an internal user. This will cover the basics of reviewing applications and should transfer to other application types.
If you have any questions or issues relating to an application, please reach out to support@conforma.nz.
Staged reviews
Application review processes come in many different formats, and can include multiple layers of review. In the Conforma Demo, the medicinal product registration process includes three stages:
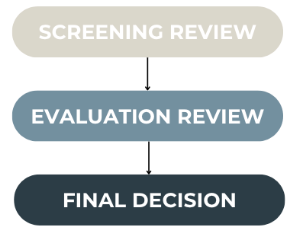
Assigning
- On the main dashboard, select Reviews awaiting assignment under Medicinal Product Registration Applications.
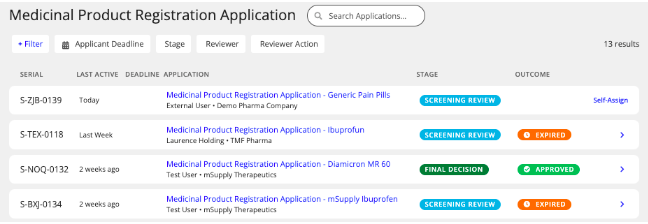
- You will be able to see a list of outstanding company registrations awaiting approval. Select the application you wish to review.
- Under the assignments tab, the reviewer for each section of the application can be selected. You can select ‘Yourself’ to self-assign the application to your workload, or assign it to the appropriate reviewer.
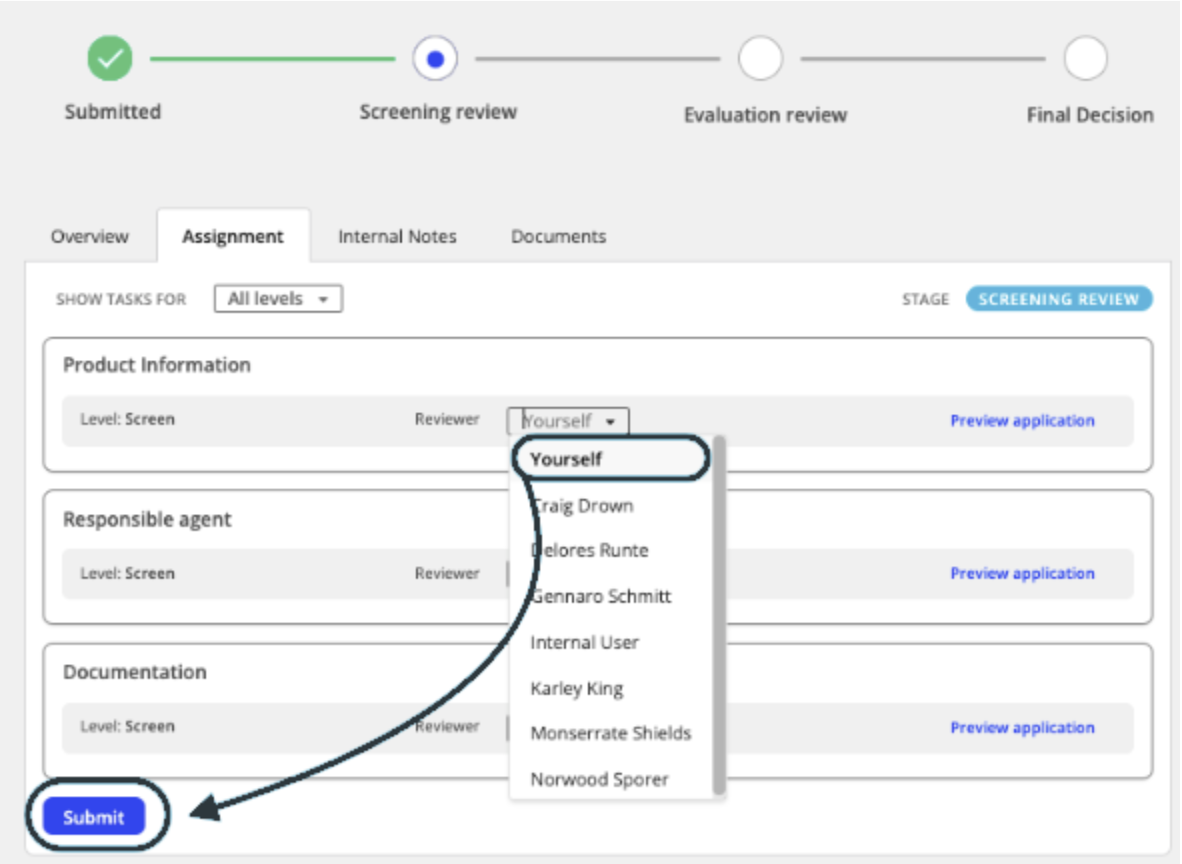
- Once you have selected the review, click Submit. The assignment will be reflected in the Assignment tab.
Reviewing the application
- Once the application has been assigned to your workload, click Start on the section you wish to review, under the assignment tab.
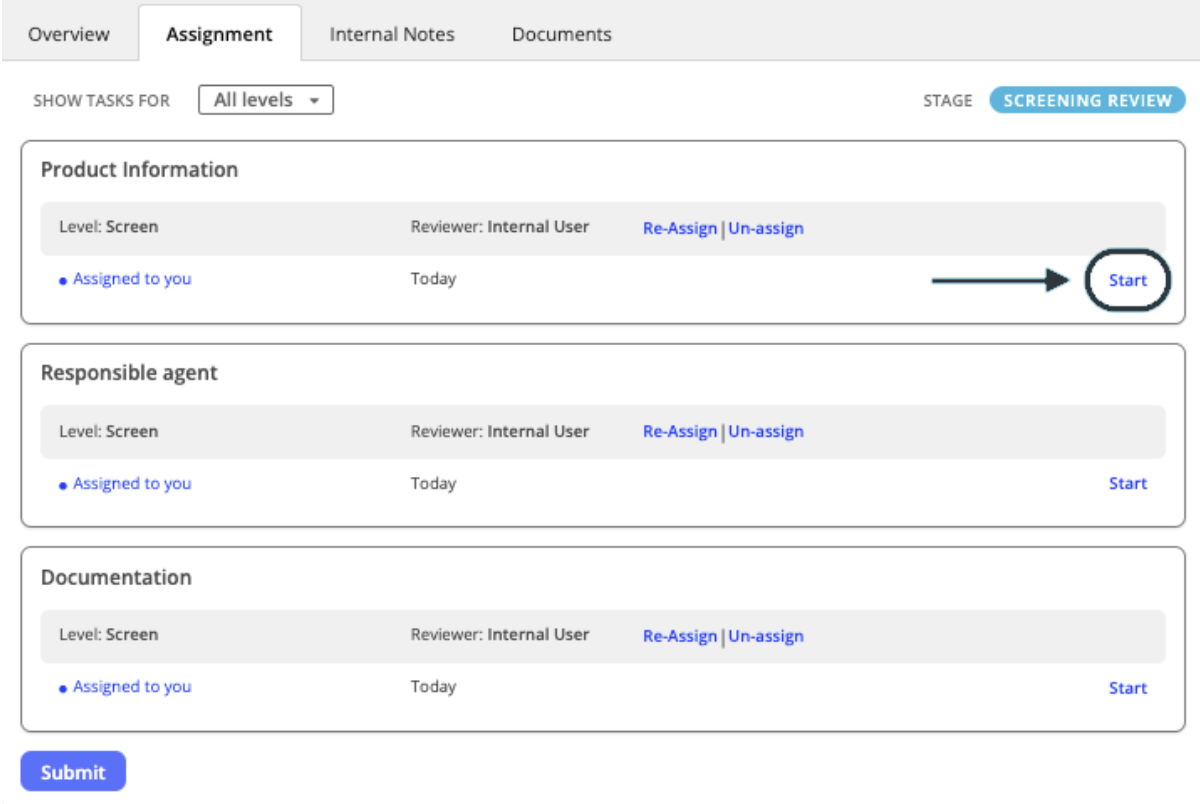
- Within each application form, all fields that have been set as assessable can be reviewed. To review an individual field, click the Review button. This opens up a space for the reviewer to mark the form requirement as either Conform (meets requirements) or Non-conform (does not meet requirements).
-
Move through the review form and mark each requirement as either a conforming or non-conforming field.
-
The comments field within the review box can be used to record internal notes as needed. Internal notes for conforming form fields are not visible to the applicant.
-
However, when a field is marked with a non-conform outcome, a comment to be communicated to the applicant is required (e.g. to provide direction to the applicant).

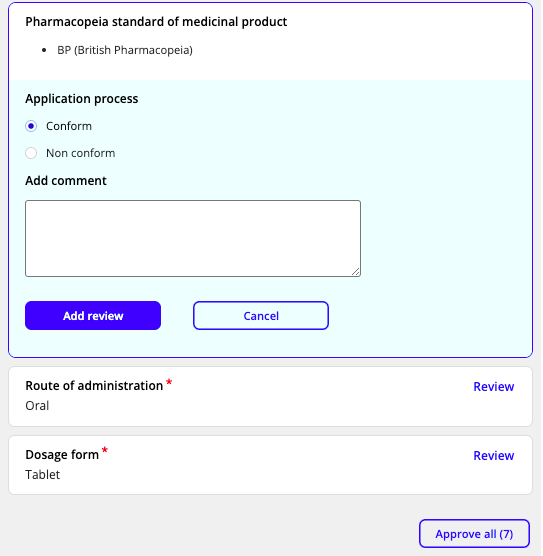
- Once you have completed your review of all of the form fields, an outcome recommendation or decision is required, depending on what stage of the review you are completing.
Conforming applications
- If every reviewable section has been marked as conforming, the only option at the end in the overall comment field will be to mark the application as ‘Conform’.
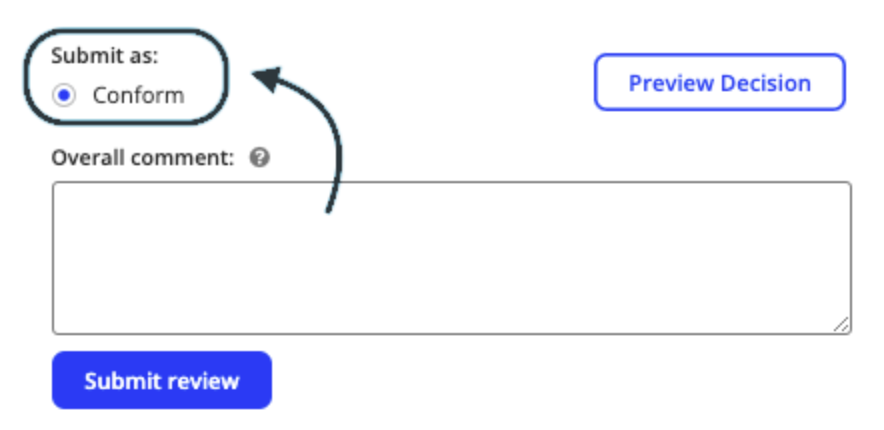
Non-conforming applications
- Where one of the review sections has been marked as ‘Non-conform’, the Overall comment section will include two options: Non-conform or Send back to applicant. If selecting Non-conform, the text included in the overall Comment field will be provided to the applicant.
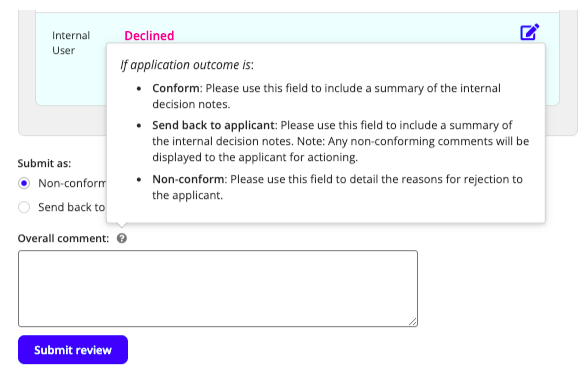
Sending applications back
-
If selecting Send back to applicant, the text included in the Overall comment field will be recorded for internal decision notes, and text included in individual review fields will be provided for the applicant.
-
Once you have selected your recommendation or decision, click Submit review.
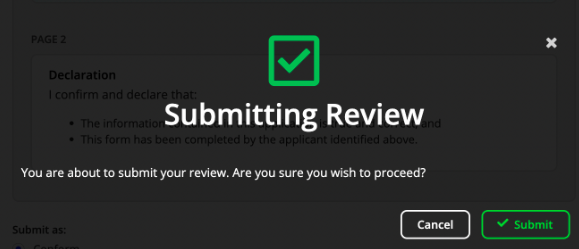
- You will be asked to confirm, choose Submit.
Progressing the application
-
If the application has multiple stages of review, it will either progress to the next stage, or be sent back to the applicant.
-
The recommendations and stages of review can be found in the Overview tab of the application.
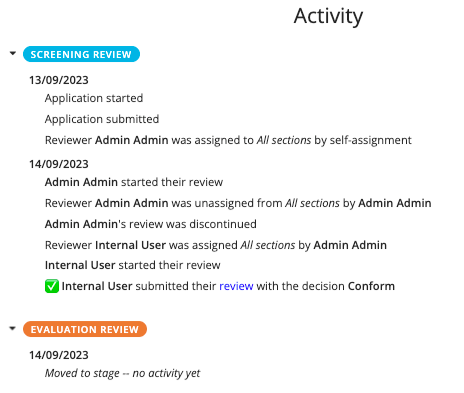
- In the example above, the screening has been completed and a recommendation made that it conforms. The application is now ready for the evaluation review and will need to be assigned again before the next stage of review can be carried out following the same process.
Final decision
-
Once all screening and evaluation stages have been completed, a final decision is required. This is assigned and initiated in the same way as any other assessment.
-
Once on the final decision stage, each section of reviews and recommendations can be viewed by selecting the drop-downs. This gives the person making the final decision a consolidated overview of the application’s review and the recommendations by the evaluators.
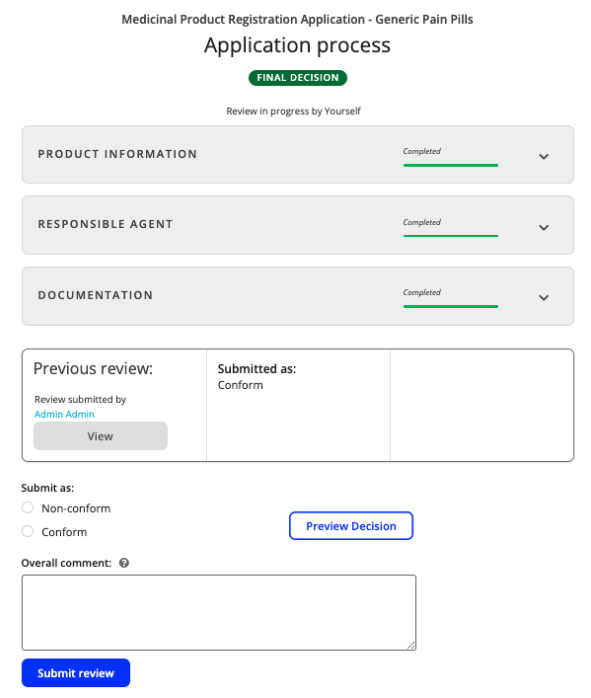
- Following this, a decision of Non-conform or Conform is required, followed by selecting Submit review.
Documentation
- Throughout each recommendation step and the final decision, you can select Preview Decision to view the documentation that will be sent to the applicant or stored internally based on your recommended outcome for the stage.
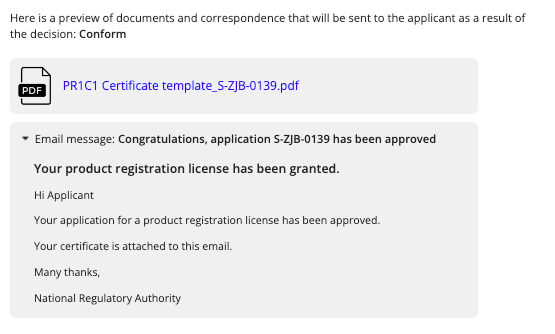
- In the case of a final decision that the application is conforming and to be approved, a product registration certificate is generated for the applicant:

Documentation, including certificates are fully customisable within the system to suit the objectives of the workflow, and everything is saved as part of the application, providing a record of decisions.