Submitting Applications
There are endless application types which can be developed in Conforma. A number of custom workflows have been developed for different Conforma users.
In this guide, we will go through the steps of a medicine application. This will cover the basics of submitting applications and should transfer to other application types.
If you have any questions or issues relating to an application, please reach out to support@conforma.nz
Submitting a new application
As noted, this example is for submitting a medicinal product registration application in the Conforma Demo, but the process is similar for all application types.
-
Ensure you are logged into your account and have selected your registered company.
-
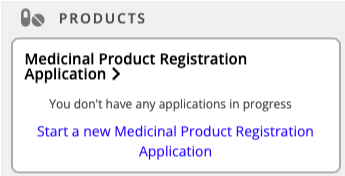
Select Start a new Medicinal Product Registration Application.
-
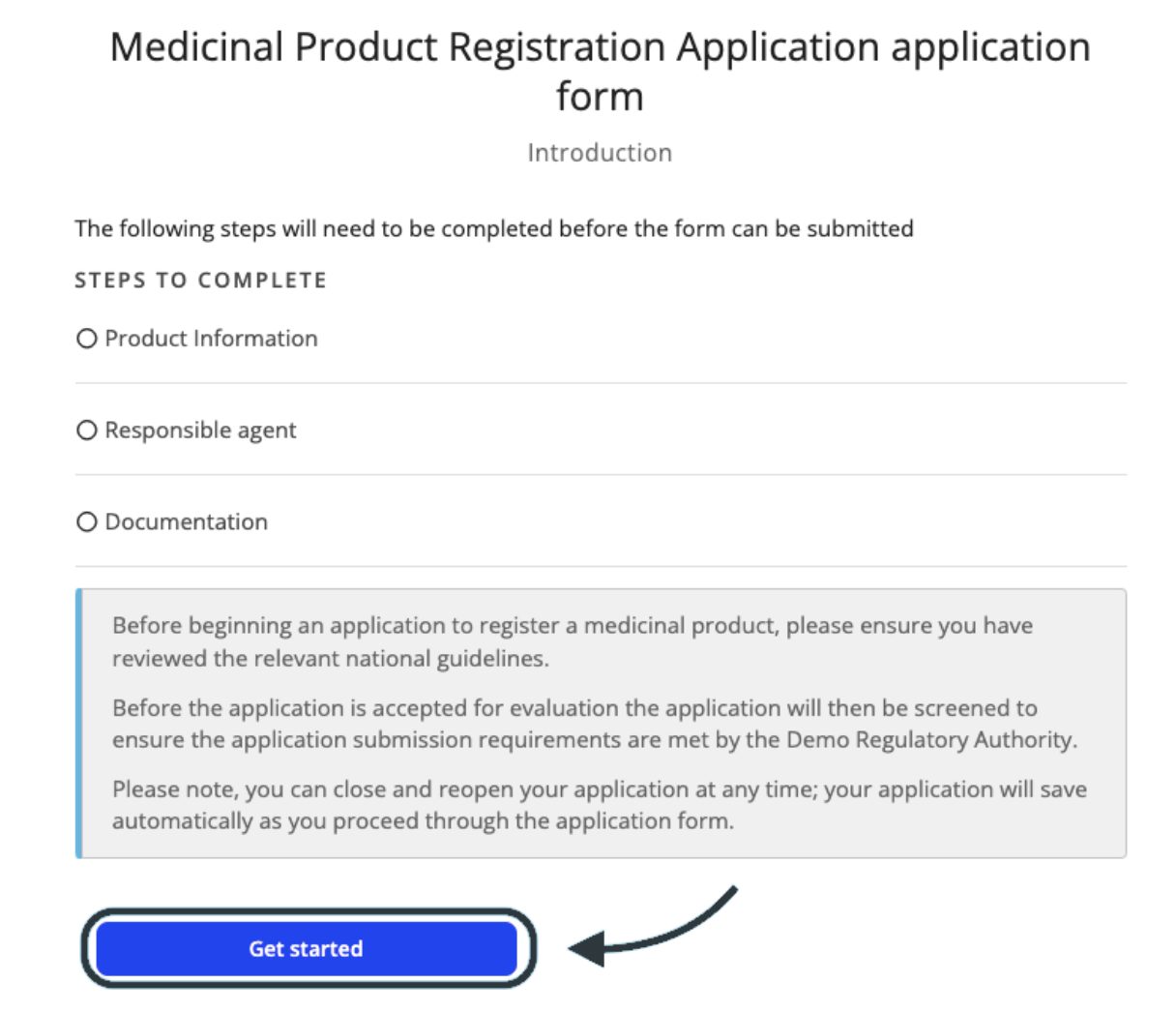
Before you start a new application, the system will provide you with an overview page to provide guidance to what information and documents are required.
-
When ready to begin the application click Get Started.
-
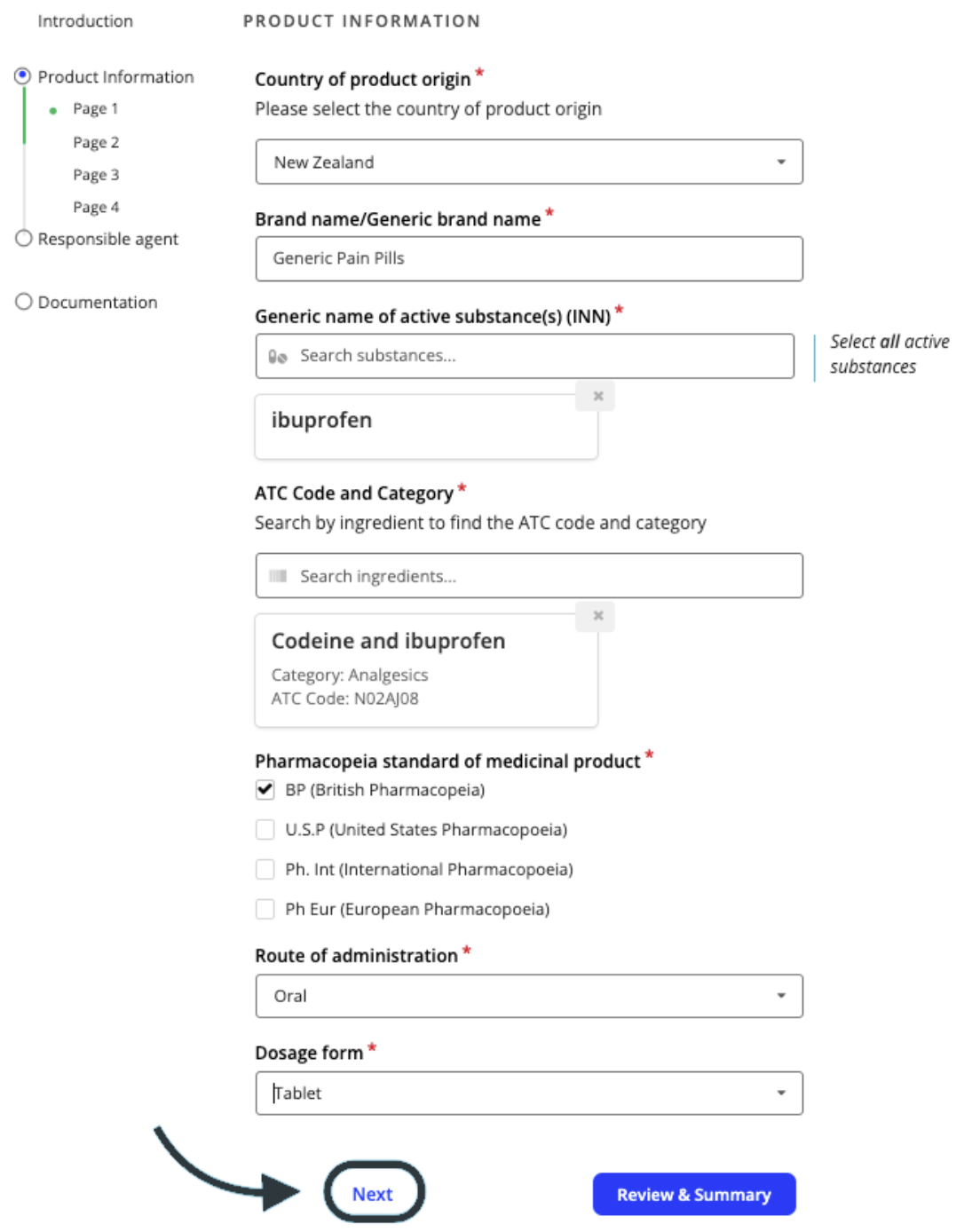
Complete the application form with the required product information. Answers to questions on the form with a red asterisk * next to it are mandatory and you won’t be able to progress the application without providing an answer.
-
Use the Next button to move through the application pages.
-
Continue progressing through the application, ensuring all required product information is included. There is a mix of free text boxes, dropdowns and checkboxes that need to be completed.
-
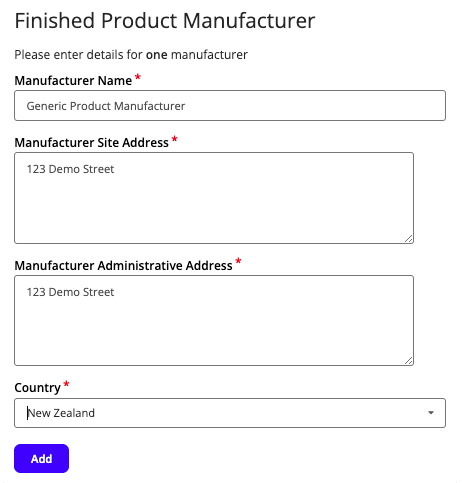
You will then need to complete details for the Associated Entities, which includes information about manufacturing sites.
-
Fill out the information for all manufacturing sites, clicking Add at the end of each form to save the information.
-
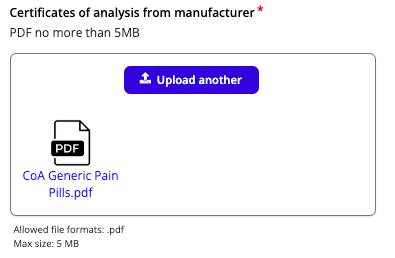
Next, you will be required to upload a copy of the requested documents. You can upload documents from your device by selecting Click to Upload. Ensure that files are named appropriately and are indicative of the document’s contents. File types and size limits are outlined below each upload box.
-
Once the form has been completed, select the Review & Summary button.
-
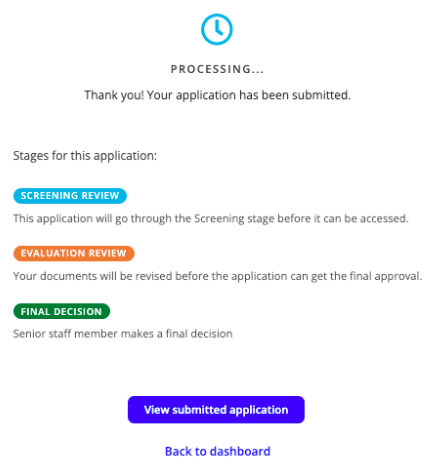
You will then be provided a summary of your completed application for final review. Once you are happy, click the Submit application button
![]()
- Once the form has been submitted, you will receive a confirmation message outlining the next steps.